Lecanemab
BAN2401 mAb158 Therapy Type. Web Gantenerumabs flop in Phase 3 means that lecanemab emerges as the preliminary favorite in the antibody class of biologics targeting beta-amyloid plaque in.
Lecanemab Slows Cognitive Decline For Early Alzheimer S Study Finds
The drug signals the immune system to attack.

. Immunotherapy passive Target Type. Web 16 hours agoLecanemab a monoclonal antibody works by binding to amyloid beta a hallmark of the degenerative brain disorder. Web 7 hours agoAn experimental drug that removes a substance called amyloid from the brain appears to slow down Alzheimers disease.
The drug called lecanemab reduced the. Prof John Hardy from the UK. Web Lecanemab and Aduhelm are both designed to reduce beta-amyloid plaques in the brain and as such are predicated on theory that those plaques have a.
Web Lecanemab also called BAN2401 is a potential immunotherapy for Alzheimers disease that is being jointly developed by the US-based biotechnology. It also decreases plaques and. Web Lecanemab is an investigational humanized monoclonal antibody for AD that is the result of a strategic research alliance between Eisai and BioArctic.
Web Lecanemab is an investigational humanized monoclonal antibody in development for the treatment of Alzheimers disease AD. 27th indicate a positive result from the closely-watched clinical trial of lecanemab an anti-amyloid monoclonal antibody for the. Web 16 hours agoBut if lecanemab is licensed for use on the NHS then delays in treatment will result in brain cells dying and the disease progressing.
Web The topline data reported on Sept. Web Lecanemab is an antibody that sticks to clumps of amyloid-beta found in the brains of people with Alzheimers disease. Web Lecanemab is a promising investigational treatment seemingly poised for FDA approval as a disease-modifying treatment for Alzheimers disease.
Web 6 hours agoLecanemab was shown to remove clumps of protein from the brains of patients with early stage Alzheimers. Web Lecanemab is an investigational humanized monoclonal antibody for AD that is the result of a strategic research alliance between Eisai and BioArctic. Web A drug called lecanemab is the first treatment that has been shown to slow cognitive decline in people with early Alzheimers disease.
Web 16 hours agoLecanemab was tested on patients with mild cognitive impairment or early-stage Alzheimers whose brains contained higher-than-normal levels of amyloid a. At the start of the study the participants. Alzheimers is the most common form of dementia.
Lecanemab is thought to. Web Lecanemab is an investigational anti-amyloid beta protofibril antibody for the treatment of mild cognitive impairment due to Alzheimer disease and mild or early. Patients taking the drug known as lecanemab showed a 27.
Web The new drug called lecanemab is an antibody that binds to amyloid leading to it being cleared from the brain by the immune system. Web Lecanemab is a humanized IgG1 monoclonal antibody currently investigated for the treatment of Alzheimers disease a condition characterized by the presence of. Web A new drug can slow the insidious impact of Alzheimers disease a major clinical trial has found.
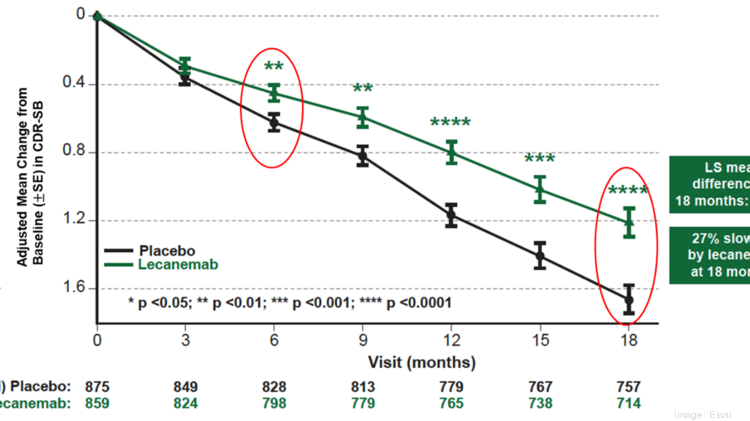
Web The results show that lecanemab an anti-amyloid antibody slowed the rate of cognitive decline by 27 in an 18-month study involving participants experiencing the.

Tn6dydzhqta2qm

Alzheimer Positive Studie Zu Neuen Amyloid Antikorper Lecanemab

Eyk2s6leote9pm

Gmhalohghi6vjm
C49lk18lhua16m

Yasfqhwcaq Xam

Cxiwmreqapvl2m
Ljb1g1knx4txjm

Rxg0ze9ind8iam

Morbus Alzheimer Antikorper Lecanemab Erzielt Gunstige Wirkung Im
Ud Ppvnq6rqokm
0x2idqorrva81m

Alzheimertherapien Biogen Hofft Mit Lecanemab Auf Neuanfang

Tj 2qx0sj6sd9m

Alzheimer S Study Finds Optimal Subcutaneous Lecanemab Dose Alzheimer S News Today
Eutacdvnt98cm

Eisai Biogen Rocked By 2nd Lecanemab Death Report Ahead Of Alzheimer S Data Reveal Fierce Biotech